Prioritize...
Prioritize...
At the completion of this section, you should be able to describe what is meant by "electromagnetic radiation" and how it is generated. You should also be able to explain the various types of electromagnetic radiation, specifically the portions of the electromagnetic spectrum that meteorologists use to observe the atmosphere. Read...
Read...
If you regularly listen to television weathercasts, you've probably heard presenters talk about "visible" satellite images. Some perplexed viewers might react by exclaiming: "Well, of course it's visible... I can see it, can't I?" What weathercasters really mean to imply, of course, is that the image of clouds that viewers see on their television screen was created by using visible light, which, by the way, is a form of electromagnetic radiation.
"Radiation?" Doesn't that pertain to things like uranium and nuclear reactors? It turns out that the scientific use of the term "radiation" is considerably more broad. Radiation is defined as the emission and transfer of energy through a medium via particles or waves. In fact, the vast majority of radiation that you encounter on a daily basis has nothing to do with nuclear radiation at all. From an everyday light-bulb, to the microwave that heats your morning coffee, to the radio that you listen to on your morning commute... all of these devices make use of different portions of the spectrum of electromagnetic (EM) radiation.
So how is this electromagnetic spectrum created? To begin with, you probably know that the building blocks of all matter are atoms and molecules. Within these atoms and molecules are smaller particles which have a specific static charge -- protons and electrons. These charged particles -- especially electrons -- tend to oscillate or vibrate. Now, physics tells us that any charged particle like an electron has an electrical field surrounding it (electrical charges and electrical fields go hand-in-hand). Furthermore, moving charges also possess magnetic fields. Thus, when an electron oscillates, its surrounding electric and magnetic fields change. Like moving your hand rapidly back and forth in a pool of water, oscillating electrons send out ripples of energy (that is, "waves") that have magnetic and electrical properties (hence, electro - magnetic radiation).

So, how is it that different kinds of electromagnetic waves exist (remember there is a whole spectrum of EM radiation)? First let's talk about the different kinds of EM radiation in terms of its wavelength. We define the wavelength of any wave as the distance between two consecutive similar points on the wave (for example from wave crest to wave crest). Now think about our pond analogy above. If you move your hand slowly in the water, you will create waves with long wavelengths. However, if you move your hand rapidly in the water, you create waves with very short wavelengths. The same is true for an oscillating electron. If the oscillation is very quick (we say the oscillation has a high frequency), then the EM radiation produced will have a small wavelength. If the oscillation is slower (having a lower frequency) then the electromagnetic waves will have long wavelengths.
Now, the frequency at which electrons oscillate is essentially set by the temperature of the matter in which the electron resides (remember we defined an object's temperature as the average kinetic energy of its atoms or molecules). The higher the temperature, the higher the frequency of oscillation. So, when temperature increases, the wavelength of the electromagnetic radiation emitted by the electron decreases. Conversely, as temperature decreases, the frequency of oscillation slows and the wavelength of the emitted electromagnetic radiation increases. Check out this simple model of how oscillation frequency is tied to the wavelength of the electromagnetic radiation.
Before leaving this discussion, let me add the following caveat: We have discussed the generation of EM radiation by a single oscillating charged molecule. In reality, matter exists as a system of charged particles. This means that the resulting electromagnetic radiation field is much more complex than I have outlined here. Remember that earlier in the course we defined temperature as the mean motion of the molecules (and that not all molecules have the same energy state). This means than a spectrum of electromagnetic radiation is generated from any system of matter that contains many charged particles, all oscillating at different frequencies. I should also note that the vibrating molecule model for electromagnetic emission only explains the existence of low-energy waves (those having lower frequencies than visible light). High-frequency EM emissions are sill generated by moving charges, but require a different mechanism to generate the high energy waves. (I discuss this in the Explore Further section if you are interested.)
Now look at the entire spectrum of electromagnetic radiation. First, note that the range in wavelengths for different types of electromagnetic radiation is staggering -- from hundreds of meters to the size of an atom's nucleus. Also note that visible light does indeed qualify as electromagnetic radiation, despite taking up only a tiny sliver of the entire spectrum. This means that we are completely blind to almost all electromagnetic radiation (most of the spectrum is invisible to the naked eye).
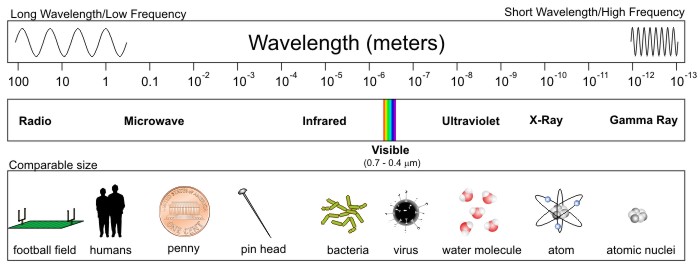
For atmospheric remote sensing we use electromagnetic radiation in the microwave, infrared, and visible bands. Perhaps most familiar to you is the visible portion of the electromagnetic spectrum. Indeed, wavelengths of EM radiation that span from approximately four tenths of a micron (1/1,000,000 of a meter) to a little more than seven tenths of a micron compose the part of the spectrum that meteorologists use to generate "visible" satellite images.
Beyond the longest wavelengths associated with visible light lies the infrared ("beyond red") band of the electromagnetic spectrum. A majority of the infrared spectrum, spanning from approximately 3 to 100 microns, essentially constitutes "terrestrial radiation" because the oscillating charges that emit at these wavelengths are consistent with temperatures commonly observed on this planet as well as the Earth's atmosphere. Thus, terrestrial radiation lends itself to be used in infrared satellite imagery (of which there are several applications).
Microwaves are next in line in the electromagnetic spectrum's hierarchy, with wavelengths spanning from 100 microns to about 30 centimeters. Most radar imagery used in weather forecasting employs artificially produced microwaves ranging in wavelength from 3 to 10 centimeters (more on radar later in the lesson).
Now that you know the terminology behind the different regions of the electromagnetic spectrum, we need to discuss the properties by which objects emit radiation. These properties have been grouped into what I will term the "four laws of radiation". Read on.
 Explore Further...
Explore Further...
If your are interested, I present below a brief discussion of the extreme short-wave portion of the electromagnetic spectrum not covered in the required reading section. If you want explore further than what I present here, there are many sources on the Internet that discuss the various regions of the electromagnetic spectrum. For starters, check out: the Wikipedia page on electromagnetic radiation.
Above the visible portion of the electromagnetic spectrum is the very short wavelength region that includes gamma rays, x-rays and ultraviolet light. The shortest wavelengths belong to gamma rays, which have wavelengths that are as short as one trillionth of a meter. To get a quantitative inkling of how small this is, consider that one meter equals 39.37 inches. So, one trillionth of a meter equals 0.000000000003937 of an inch. Wow! That's a pretty short distance from trough to trough in a wave. The energy required for matter to emit electromagnetic radiation with wavelengths on the order of a few microns (or less), surpasses that which can generated by an oscillating molecule. In fact, at such energies, the molecular and atomic bonds may breakdown completely, leaving only single atoms (or even single electrons!). Therefore, a few new mechanisms are needed to explain very short-wave EM emissions.
Perhaps you remember the Bohr model of an atom from high school chemistry that shows the electrons orbiting a nucleus of protons and neutrons (like a mini solar system). Suffice to say, things are a bit more complicated than that, but I'll stick with this model for simplicity. In an unenergized state (called the base state), an atom has a number of electrons in various orbits (or shells) around the nucleus. However, if sufficient energy is added to the atom, one or more of its electrons will be ejected into to higher orbits around the nucleus (because they have more energy, they can better overcome the pull of the nucleus). Then when these electrons fall back down to their original orbit, they must jettison the extra energy. They emit this energy in the form of a photon (a small packet of EM radiation), that has a frequency which corresponds to the energy released. Such photons are typically found in the near-IR, visible, and ultraviolet portions of the EM spectrum.
At even higher temperatures, the electrons may even break their bonds with the atomic nucleus itself, forming a what is known as a plasma. Plasmas are a fourth state of matter (not a solid, liquid, or gas) that consist of positive ions (left over atomic nuclei) and free electrons. In a plasma, electromagnetic radiation is generated when the speed or direction of an electron is altered by a positive ion or another electron. Because of the unrestrained nature of electrons within a plasma, they can travel at tremendous speeds and thus can generate very high-energy photons. The generation of ultraviolet waves, X-rays,and gamma rays are typically from plasmas.
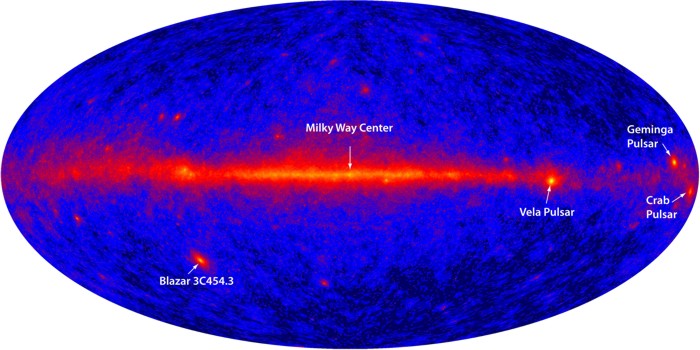
Although such high-energy radiation can be generated artificially (the medical use of X-rays, for example), most of the sources for natural high-energy EM emission originates in space. The plasma of our sun emits copious amounts of X-rays and ultraviolet radiation, as well as gamma rays during eruptions of solar flares. Furthermore, the most prodigious gamma-ray bursts come from interstellar events such as supernovae, black holes, and quasars. Check out the image below which shows gamma ray emission from the entire sky. Note that the strongest gamma ray emissions are concentrated along the disk of the Milky Way Galaxy.